X
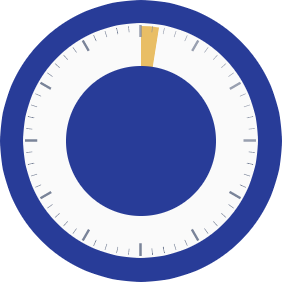
A single dose of low-viscosity IHEEZO™ was shown to sustain sufficient ocular surface anesthesia for the duration of routine cataract surgery, lasting an average of 21.5 minutes.1
Rapid onset of less than 90 seconds to achieve sufficient anesthesia
 <90
<90
Many typical ophthalmic procedures last 10 to 15 minutes
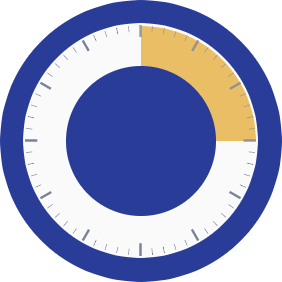 10-15
10-15
Sufficient anesthesia with IHEEZO lasted an average of 21.5 minutes
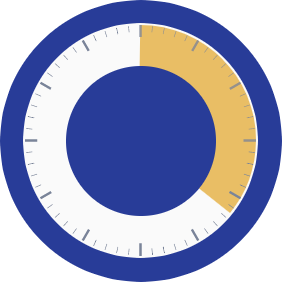 21.5
21.5
Rapid onset of less than 90 seconds to achieve sufficient anesthesia
Many typical ophthalmic procedures last 10 to 15 minutes*
Sufficient anesthesia with IHEEZO lasted an average of 21.5 minutes
*The Phase III clinical trial was a randomized, prospective, multicenter, active-controlled, observer-masked study in 338 patients undergoing routine cataract surgery.
No patient treated with IHEEZO in this clinical trial required supplemental treatment to maintain anesthesia.†
In the clinical trials, the most common adverse reactions following IHEEZO administration (n=151) were2:
Mydriasis (26%)
Conjunctival hyperemia (11%)
Eye irritation (6%)
IHEEZO is contraindicated in patients with a history of hypersensitivity to any component of this preparation.2
IHEEZO is a unique formulation of chloroprocaine, which has an established safety profile comparable to other local anesthetics.1
IHEEZO is preservative-free, and delivered in a sterile, single-use ampule for added patient safety.3
Surgeons reported that they felt confident using IHEEZO in the clinical trial, calling it “uncomplicated” at each time point of the study.1
Sufficient ocular surface anesthesia with IHEEZO in routine ocular procedures may help reduce the need for intraoperative opioids.1,4
†In the clinical trial, no patient undergoing routine cataract surgery receiving IHEEZO required supplemental treatment to maintain anesthesia; this was not the case for patients receiving tetracaine. Supplemental treatment was defined as general anesthesia. Though supplemental administration was not required by any patient in the clinical trial, IHEEZO may be reapplied as needed to maintain anesthesia.1
IHEEZO is indicated for ocular surface anesthesia.
IHEEZO is contraindicated in patients with a history of hypersensitivity to any component of this preparation.
IHEEZO should not be injected or intraocularly administered.
Patients should not touch the eye for at least 10 to 20 minutes after using anesthetic as accidental injuries can occur due to insensitivity of the eye.
Prolonged use of a topical ocular anesthetic may produce permanent corneal opacification and ulceration with accompanying visual loss.
Do not touch the dropper tip to any surface as this may contaminate the gel.
IHEEZO is indicated for administration under the direct supervision of a healthcare provider. IHEEZO is not intended for patient self-administration.
The most common adverse reactions in studies following IHEEZO administration (incidence greater than or equal to 5%) were mydriasis, conjunctival hyperemia, and eye irritation.
You are encouraged to report suspected adverse reactions to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
Please see the Full Prescribing Information for IHEEZO at www.iheezo.com/prescribinginformation.
References: 1. IHEEZO. Prescribing information. Harrow IP, LLC; 2022. 2. Data on File. Harrow IP, LLC; 2023. 3. Chloroprocaine hydrochloride ophthalmic gel 3%. Pharmaceutical development. Sintetica; 2022. 4. Han J. Rinella NT, Chao DL. Anesthesia for intravitreal injection: a systematic review. Clin Ophthalmol. 2020;14:543-540.
IHEEZO is indicated for ocular surface anesthesia.
IHEEZO is contraindicated in patients with a history of hypersensitivity to any component of this preparation.
IHEEZO should not be injected or intraocularly administered.
Patients should not touch the eye for at least 10 to 20 minutes after using anesthetic as accidental injuries can occur due to insensitivity of the eye.
Prolonged use of a topical ocular anesthetic may produce permanent corneal opacification and ulceration with accompanying visual loss.
Do not touch the dropper tip to any surface as this may contaminate the gel.
IHEEZO is indicated for administration under the direct supervision of a healthcare provider. IHEEZO is not intended for patient self-administration.
The most common adverse reactions in studies following IHEEZO administration (incidence greater than or equal to 5%) were mydriasis, conjunctival hyperemia, and eye irritation.
You are encouraged to report suspected adverse reactions to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
Please see the Full Prescribing Information for IHEEZO at www.iheezo.com/prescribinginformation.
References: 1. IHEEZO. Prescribing information. Harrow IP, LLC; 2022. 2. Data on File. Harrow IP, LLC; 2023. 3. Chloroprocaine hydrochloride ophthalmic gel 3%. Pharmaceutical development. Sintetica; 2022. 4. Han J. Rinella NT, Chao DL. Anesthesia for intravitreal injection: a systematic review. Clin Ophthalmol. 2020;14:543-540.